Building partnerships to realize ATP citrate lyase inhibitory potential in cardiovascular disease and beyond, translating innovation into meaningful therapies.
At Esperion, we partner with companies that have proven expertise and passion for evolving scientific progress while addressing unmet patient needs. Our established medicines offer new, innovative options for patients struggling with their LDL-C. We believe partnering with strong commercial organizations around the world is the best way to enable broad access to our medicines globally.
Esperion continues to build on its success with bempedoic acid by developing a series of novel Next Generation ATP-citrate lyase inhibitors (ACLYi). Our leading-edge approach to drug discovery integrates recent insights into the structure-function of ACLY, and its newly discovered role as a pathway nexus linking metabolism, immunity, inflammation and fibrosis. Our Next Generation drug discovery program has led to the development of highly potent, specific, and differentiated allosteric inhibitors optimized to address a variety of liver and kidney diseases.
Partnering opportunities:
We are seeking to accelerate some of these parallel programs with strategic partnerships.
There is significant opportunity, with the right partner, to discover and develop next gen ACLY inhibitor(s) that are optimized to beneficially modify multiple distinct diseases.
Established Partnerships
Our established partnerships to commercialize bempedoic acid and its combination with ezetimibe in Europe and Japan represent our largest agreements.

We have partnered with Daiichi Sankyo Europe to commercialize our two marketed products in the European Economic Area, UK, Turkey and Switzerland. Nilemdo® (bempedoic acid) Tablet and Nustendi® (bempedoic acid and ezetimibe) Tablet have launched in Germany, Switzerland, Austria, Belgium, Luxemburg, Netherlands, Spain, Italy, Slovakia, and Czech Republic with continued launches planned throughout the European Union.
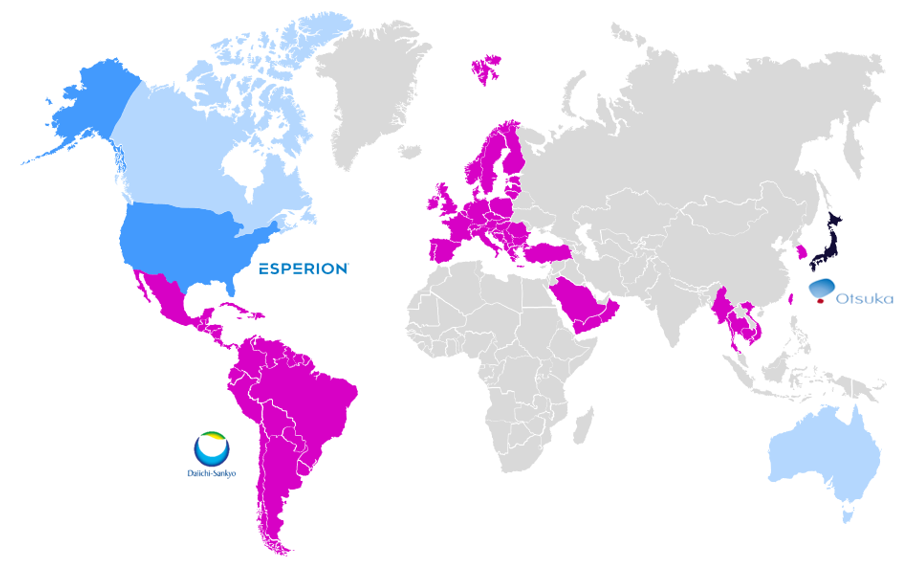

In April 2021, we further expanded our partnership with Daiichi Sankyo, granting Daiichi Sankyo exclusive commercialization rights to bempedoic acid and the bempedoic acid & ezetimibe combination tablet in S. Korea, Taiwan, Hong Kong (products launched in September 2023), Thailand, Vietnam, Brazil, Macao, Cambodia and Myanmar. The terms allow for potential expansion across geographies in the Middle East and Latin America.

In Japan, have partnered with Otsuka Pharmaceutical Company to continue to develop and commercialize bempedoic acid and its combination with ezetimibe. The total value of the agreement is $600 million including milestones and development costs, and Otsuka will pay Esperion a significant royalty on all net sales. Otsuka will manage all ongoing development, regulatory approvals, and commercialization activities in Japan. Otuska plans to file for approval in 2024 with anticipated NHI pricing in 2025.
Partnering opportunities
Esperion continues to build on our success with bempedoic acid in our Next Gen program which is focused on developing ATP citrate lyase inhibitors (ACLYi). New insights into the structure and function of ACLY fully enables rational drug design and the opportunity to develop highly potent and specific inhibitors with allosteric mechanisms. We are seeking to accelerate some of these parallel programs with strategic partnerships.
Reach out to our business development team to learn more about current partnership opportunities with Esperion.